|
||||||||||||
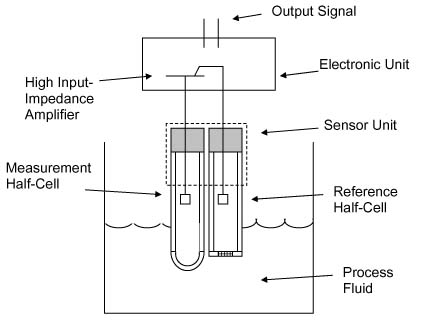
E-Zine November 2006Systems used to measure pH and ORP provide the ability to determine the concentration of a specific ion or group of ions in solution over an incredibly wide range and with great sensitivity. However, they are beset with a number of theoretical and practical pitfalls that often make their application difficult for both end users and suppliers. The ions that pH and ORP measurement systems detect are important to a number of large industries such as wastewater treatment, water treatment, chemicals, food, pharmaceuticals, pulp and paper, metal plating and power generation. In these industries, there are often significant economic incentives to measure these concentrations continuously rather than to manually sample the process and perform periodic offline analyses. Due to the wide range of issues that can introduce uncertainty into these measurements, practitioners have typically reverted to a trail-and-error approach to determine the type of analyzer to use. This often results in significant delays in identifying a measurement system that meets the application requirements or, worse yet, resorting to manual sampling and analysis. One side effect of this state of affairs is a tendency to view the entire pH/ORP measurement system selection process as more art than science and more wizardry than art. This report is written from a process instrumentation end user perspective in an attempt to reduce the uncertainty associated with the application and operation of these measurement instruments. The approach used in this report is based on the general principles described in international standard IEC 60359 (Electrical and Electronic Measuring Equipment-Expression of Performance). This approach focuses on describing the both the uncertainty under reference conditions (intrinsic uncertainty) and the uncertainty under actual operating conditions (operating uncertainty). This approach has proven to be effective for similar process measurement instrumentation. International standards IEC 60746-2 (Expression of Performance of Electrochemical Analyzers, Part 2: pH Value) and IEC 60746-5 (Expression of Performance of Electrochemical Analyzers, Part 5: Oxidation Reduction Potential or Redox Potential) address the performance of pH and ORP measurement systems, respectively. As per IEC 60746, a pH or ORP analyzer consists of a sensor unit and an electronic unit as shown in the figure below.
These devices are called by different names in different parts of the world and in different technical communities as illustrated in the table below.
The electronic unit generates the measurement output signal that is used to monitor and/or control the process. However, the performance and long-term stability of this signal are primarily determined by the design of the sensor unit. Therefore, this report tends to emphasize the sensor unit and its effect on the measurement. This article was excerpted from The Consumer Guide to Industrial pH and ORP InstrumentationThe Consumer Guide to Differential Pressure Flow Transmitters ISSN 1538-5280 |
||||||||||||