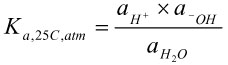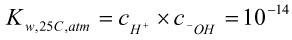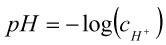|
||||||||||||||||||||||||||||||||||||||||||||||||||
E-Zine December 2006The concepts of pH and ORP require some explanation because they tend to be more abstract than temperature, pressure and flow rate. Both pH and ORP measurements are primarily made in aqueous solutions so a reasonable understanding of these measurements requires some knowledge of the chemistry of water. Water is a molecule consisting of two hydrogen atoms bound to a single oxygen atom. Water reversibly dissociates into a hydrogen ion, H+, and a hydroxyl ion, OH- as shown below.
Positively-charged ions like the hydrogen ion are called cations and negatively-charged ions like the hydroxyl ion are called anions. Since pH is a measurement pertaining to H+ ions and ORP is a measurement pertaining to other ions in solution, it is necessary to understand how these ions influence the properties of their solution. All ions in solution interact with each other. As their concentration increases, the types of interaction grow more complex and the magnitude of these interactions increases. Since the properties of ionic solutions are a function of more than just ion concentrations, the solution properties are best expressed in terms of activities. The relationship for the hydrogen ion is:
The activity coefficient provides a means to account for interactions with other ions in solution. One property of solutions that can be described using activities is the state of equilibrium. When equilibrium is established for pure water at 25°C and atmospheric pressure, the relative proportions of molecular water, hydrogen ions and hydroxide ions can be written in terms of an acid constant,
Since the water molecule dissociates only slightly and, for dilute solutions, the activity of the water molecule is relatively constant, the activity of water can be combined with the acid constant to create the dissociation constant, Kw. For dilute solutions, the ion activity coefficients are approximately one so the dissociation constant is often expressed in terms of ion concentrations as shown below.
The term pH is defined as
For dilute solutions, the activity coefficient is approximately one, so this simplifies to:
For purified water, the concentration of hydrogen ions equals the concentration of hydroxide ions so
Pure water is considered neutral at a pH of 7. Most pH measurement applications do not involve pure water but the dissociation of molecular water still occurs when other substances are added to water. The table below demonstrates the hydrogen ion and hydroxide ion concentrations that would exist in dilute solutions of various pH values.
A substance with a pH less than 7 is considered acidic whereas a substance with a pH higher than 7 is considered basic. Negative pH values and values greater than 14 (although rare) are possible. The simplifying assumptions cited above for dilute solutions are only valid at molar concentrations less than 0.001 g-mole per liter. Values in the previous table that exceeded this concentration are shown in parentheses. ISSN 1538-5280 |
||||||||||||||||||||||||||||||||||||||||||||||||||


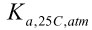 , and the activity of each of the species as shown below.
, and the activity of each of the species as shown below.